TGA UPDATE
TGA UPDATE ON BREAST IMPLANT AND TISSUE EXPANDERS
The Therapeutic Goods Administration (TGA) has completed its review into breast implants and breast tissue expanders following reports of around 100 cases of Breast Implant Associated Anaplastic Large Cell Lymphoma in Australia (BIA ALCL), including four deaths. The TGA has decided to take regulatory action on all breast implant and breast tissue expanders currently included in the Australian Register of Therapeutic Goods (ARTG).
Eight models of breast implants are to be suspended from supply in Australia for six months, while a number of safety and performance concerns are addressed. Any stock of these un-implanted devices in the market will also be recalled during the suspension period. No models of breast tissue expanders have been suspended.
In addition to the suspensions, two industry sponsors have cancelled the supply of their highly textured implants and tissue expanders since the review was initiated.
All other breast implant and tissue expander devices that have not been suspended will require strict conditions of supply to be met.
The TGA will be holding two patient/consumer and clinician forums next Wednesday in conjunction with the Breast Cancer Network Australia and the Cancer Council to explain the decision and be further guided in communication approaches.
Australian Society of Plastic Surgeons (ASPS) is pleased with this result. It is the outcome we advocated for when we met with the TGA last month and argued against a blanket ban of grade 2, 3 and 4 implants.
SUSPENSION AND RECALL
Suspended implants are those that are “macrotextured” – grade 3 and 4 and some “microtextured” implants associated with higher incidences of BIA-ALCL and other clinical concerns.
WHICH PRODUCTS HAVE BEEN SUSPENDED?
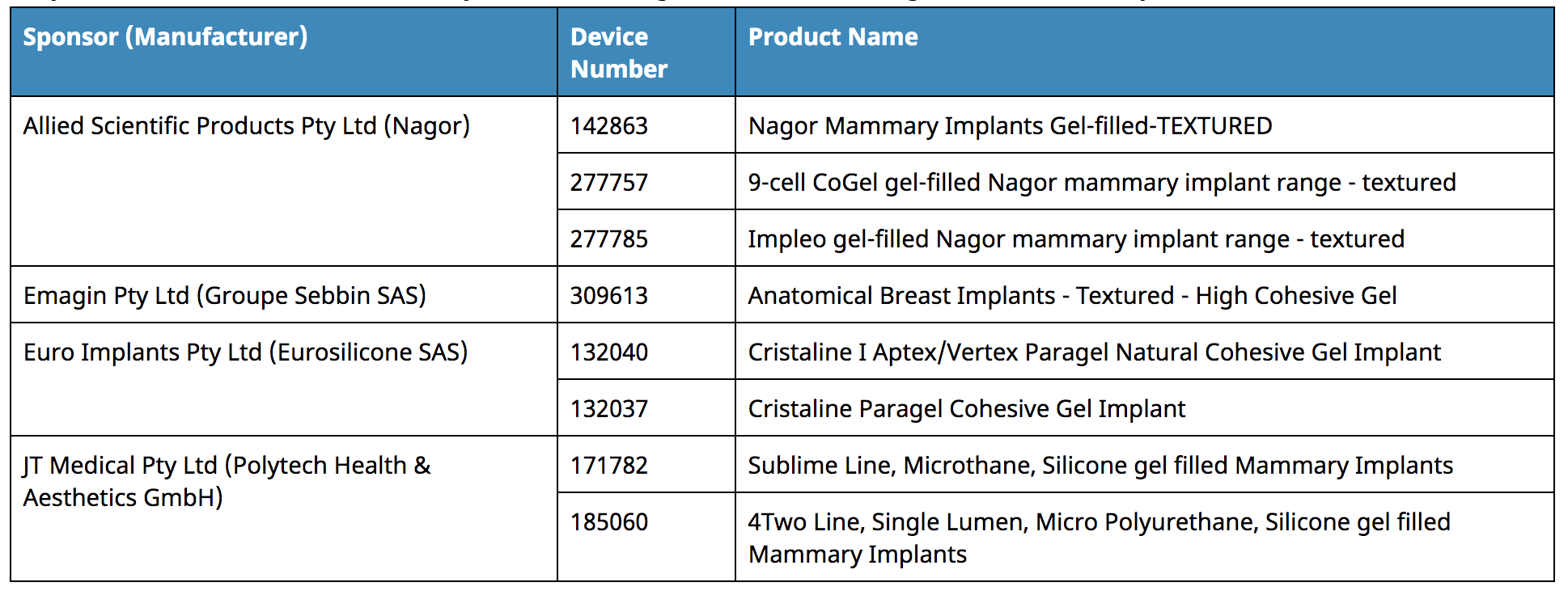
- The ARTG inclusion of the above breast implant devices is suspended.
- The devices will no longer be able to be supplied, imported, or exported from Australia for the duration of the suspension (6 months), effective 25 October 2019.
- A suspension can be revoked if concerns about the devices are addressed to the TGA’s satisfaction.
- If concerns persist, the suspension may be extended or the entries of the devices may be cancelled.
- All un-implanted products will be subject to recall to remove them from the market (i.e. on hospital shelves).
BACKGROUND
The evidence available at present indicates that while causes are likely to be multi-factoral BIA-ALCL is more likely to occur in implants with a greater surface area and roughness of their wall. The degree of texturing in the surface is a result of the materials used in the production of the device or the manufacturing processes.
The TGA is taking a precautionary approach and suspending particular breast implant devices which have a higher degree of texturing, or a higher risk of BIA-ALCL, or deficiencies in evidence to demonstrate the manufacturer has adequate risk detection and mitigation measures in place.
Over the past few years there has been a change in the type of breast implants used in Australia with a decrease in macro-textured implants and an increase in supply of micro-textured and smooth implants. The suspensions imposed by the TGA do not impact on the supply of the smooth implants available and to date there are no known cases of BIA-ALCL in Australia where only smooth devices were implanted.
THE EXPERT ADVISORY PANEL HAS PROVIDED THE FOLLOWING Q&A’S
What is breast implant-associated BIA-ALCL?
Breast implant-associated ALCL is a rare type of cancer.
It usually involves a swelling of the breast, typically 3 to 14 years after the operation to insert the breast implant. This swelling is due to an accumulation of fluid. Breast implant-associated ALCL has been known to occur as soon as 1 year after the operation and as late as 37 years after the operation.
Less commonly, breast implant-associated ALCL can take the form of a lump in the breast or a lump in the armpit.
If you notice any of these problems (swelling or a lump), or have any other concerns with your implants, you should seek medical attention.
Most cases of breast implant-associated ALCL are cured by removal of the implant and the capsule surrounding the implant.
What is the risk?
Based on the currently available data, it is not possible to provide an accurate estimate of risk. Current expert opinion puts the risk of ALCL at between 1-in-1,000 and 1-in-10,000. Based on currently available data, most (95%) of cases of breast implant-associated ALCL occur between 3 and 14 years after the implant (median: 8 years; range: 1-37 years).
It can be difficult to express this risk in a concrete way, such that you can make a fully informed decision about whether or not to have a breast implant. Some different ways of expressing the risk are given below:
One woman will be diagnosed with breast implant-associated ALCL for every 1000 to 10,000 women with breast implants.
Suppose we took 1-in-5000 women, the middle of the experts’ range, as the best estimate of risk of ALCL in women who have breast implants. This would mean that, of 5000 women with implants, one woman will develop ALCL over a period of about 3-14 years following an implant; the other 4999 women will not develop ALCL.
Should implants be removed, just in case?
Because breast implant-associated ALCL is rare, experts do not recommend removal of breast implants for women who have no problems with the implant. If you are concerned you should discuss your options with your doctor.
Generally, breast implants are not lifetime devices regardless of breast implant-associated ALCL. Typically they are removed after 10-15 years. The longer you have the implant, the more likely it will need to be removed. Common reasons for removal are contracture (hard or painful implants) or movement of the implant.
How is breast implant-associated ALCL diagnosed?
If you develop swelling of an implanted breast your doctor will send you for an ultrasound scan to see if this is due to a fluid collection. If fluid is present it will be removed and sent to the laboratory for analysis. Most fluid collections are not ALCL, but the laboratory test will be able to tell for sure. Mammograms are not helpful for diagnosing ALCL.
Other investigations such as MRI and CT-scans would typically be done if the laboratory analysis of the fluid confirms a diagnosis of ALCL.
Should women with implants be screened for ALCL?
Based on external expert clinical advice received by the TGA, regular screening is not recommended at this time.
If you notice enlargement or swelling of one or both breasts, or a lump, you should seek medical advice as soon as possible.
Are some women more at risk of breast implant-associated ALCL than others?
Breast implant-associated ALCL can develop regardless of whether the implant is inserted for cosmetic reasons or for reconstruction of the breast following breast cancer.
It can occur with both saline and silicone gel filled implants.
To date:
- No Australian cases have been reported in women who have only had smooth implants.
- All Australian cases have occurred in women who have had textured or polyurethane implants.
Based on the currently available data:
- It is uncertain whether textured (either micro or macro) and polyurethane implants carry different risks.
- It is uncertain whether different brands of textured and polyurethane implants carry different risks.
- It is not possible to predict which women with textured or polyurethane implants will develop breast implant-associated ALCL.
What is the prognosis and treatment of breast implant-associated ALCL?
Most cases are cured by removal of the implant and capsule surrounding the implant. Usually your doctor will remove both implants, even if breast implant-associated ALCL has only occurred in one breast. This is because there is a small but real risk that breast implant-associated ALCL can develop in the opposite breast. Sometimes there is a solid lump (not just fluid). In these cases, chemotherapy or radiotherapy may be required.
Over the last 10 years, three Australian women have died from breast implant-associated ALCL.
The management of breast implant-associated-ALCL is multidisciplinary with all patients requiring a referral to a surgeon experienced with breast implants and the involvement of a haematologist who specialises in lymphoma, for initial and ongoing investigations and management.
What has the TGA been doing to monitor BIA-ALCL?
The TGA has been monitoring BIA-ALCL since 2011 after receiving the first adverse event report in 2009. It established an expert working group in 2016 to seek advice on BIA-ALCL. The working group includes plastic surgeons, cosmetic surgeons, breast-cancer surgeons, cancer epidemiologists, data analysts, public -health practitioners and consumers.
The TGA has worked with this group to develop targeted information for consumers and health professionals.
The introduction of a new requirement for Patient Information Leaflets for implantable devices commencing in 2018 enhances patient access to information.
Why hasn’t TGA immediately banned these products?
There are a range of types of textured breast implants supplied in Australian. By compelling suppliers to provide additional information specific to the Australian market, the TGA can make well informed decisions about safety of particular types of implants. Suppliers have 10 working days to respond to the request for information. After receiving this information the TGA will consider action to suspend or cancel particular products.
The decision by the French regulator appears to be based on events described to them from patients and doctors as well as data published about three implants, which has been extrapolated to other products. Other regulators, including those in the US, Germany and the UK are also seeking additional evidence on the risk of BIA-ALCL, and we are working closely with these regulators.
Patients who have breast implants should expect that the regulator makes decisions based on sound scientific and medical evidence.
Textured implants of varying roughness are used in 82% of operations in Australia. They play an important role in reconstructive surgery. Smooth implants are an alternative but they may require a higher rate of replacement reoperation due to greater rates of contracture of these implant.
The international consensus is that there is no evidence supporting the removal of breast implants in the absence of properly diagnosed BIA-ALCL.
A MESSAGE FROM DR FARHADIEH:
WHAT SHOULD YOU DO?
1) Find out what implant type you have.
2) Contact your surgeon; we routinely follow up our patients and contact them in any cases of notification. We do not use Allergan implants.
3) If you are considering implant surgery, do your research and make sure that you find a qualified specialist plastic surgeon and ask questions.
Please contact us today if you would like more information.
ASK OUR TEAM
Here at Panthea, we are dedicated to being there for every step of the journey with you. Get in touch with our Sydney or Canberra team if you would like additional information regarding breast implant-associated ALCL.










