BREAKING NEWS: ALLERGAN RECALLS TEXTURED IMPLANTS
Within the past 24 hours, Allergan has announced a worldwide recall of textured breast implants after the Food and Drug Administration (FDA) found an increase in a rare cancer and deaths linked to the textured implants. The FDA decision, based on an increasing number of cases and deaths from the implant-associated cancer, lags far behind action in Europe, where the Allergan devices were effectively banned late last year.
“Patient safety is a priority for Allergan,” the company said in a statement, “and patients are advised to speak with their plastic surgeon about the risk and benefits of their implant type should they have any concerns.”
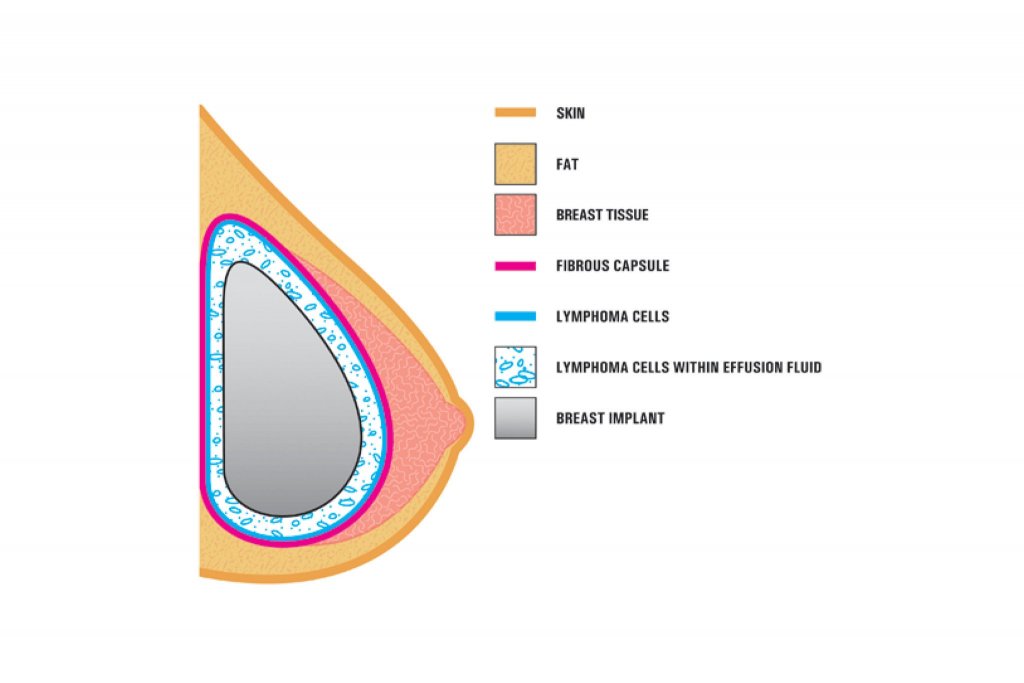
A diagram from the F.D.A. showing how the cancer forms around the implant. The lymphoma is usually found near the breast implant, contained within the fibrous scar capsule or in the fluid surrounding the implant. The disease is not in the breast tissue itself. Researchers have not been able to explain the link between the textured implants and the rare cancer.
This was not unexpected from Dr Farhadieh’s perspective, as the clinical and basic science research has been pointing to this for some time. The textured implants have been implicated in development of a rare but highly treatable type of lymphoma called Anaplastic Large Cell Lymphoma (BIA-ALCL). BIA-ALCL develops around breast implants and occurs most frequently in patients who have breast implants with textured surfaces. This is a cancer of the immune system, not a type of breast cancer. When caught early, BIA-ALCL is usually curable.
The first reported case of a patient with BIA-ALCL was in 1997. 5 women in Australia and New Zealand have died of BIA-ALCL and 107 Australian women have been diagnosed with BIA-ALCL. There have been 4 related cases of death. Explantations (removal of the implant and capsule) included higher surface area of implants. 11 patients had complications of either rupture, contracture or infection. All patients underwent total capsulectomy and resection of capsule. The most significant point here is that they were all textured implants and this seems to be the reason for Allergan’s withdrawal of all textured implants and tissue expanders. The current recommendation involves analysing any new seromas for possibility of this.
The Australian Therapeutic Goods Association (TGA) has also announced a proposal to ban Allergan textured implants.
A MESSAGE FROM DR FARHADIEH:
Individuals who have breast implants should be made aware of the risks associated with Allergan textured implants. We encourage you to contact your surgeon to find out what implant type you have. We have already had multiple patients reach out to us since TGA announcement who received textured implants from their surgeons.
If you are considering implant surgery, do your research and make sure that you find a qualified specialist plastic surgeon and ask questions.










